In the study of metal-catalyzed asymmetric reactions, chiral ligands are a key factor affecting the stereoselectivity of the reaction. Therefore, chiral ligands with simple design structure, convenient synthesis, and high catalytic selectivity are organic chemists in the field of asymmetric synthesis A topic that we have been paying attention to. In the past 30 years, although great progress has been made in this area, some chiral ligands with excellent catalytic selectivity have been invented, but most of the structures are complicated, the synthesis steps are lengthy, the development of novel structures and simple frameworks Chiral ligands are still challenging.
Combined with the research accumulation of chiral sulfur chemistry and chiral olefin chemistry in the work of the past few years, Xu Minghua's group of Shanghai Institute of Materia Medica, Chinese Academy of Sciences recently proposed a new chiral thioene based on sulfur chirality with a simple open-chain molecular skeleton Ligand-like concept. Compared with the reported chiral olefinic ligands, chiral thioene compounds do not need to build a complex bridge ring or bicyclic carbon chiral skeleton, the synthesis is simpler; and because the inherent sulfur chirality in the molecule is directly connected to the metal catalytic center, May be more conducive to the stereoselectivity of the reaction.
The study found that the two types of simple chiral thioene compounds designed, namely chiral sulfenamide-ene and chiral sulfoxide-ene, can form complexes with metal rhodium. Symmetrical 1, 4 addition shows high catalytic activity and excellent enantioselectivity. Relevant research results were published in the Chem. Commun. (2011, 47, 7230-7232) and the Organic Letters (2011, 13, 3410–3413) of the Royal Society of Chemistry. These two works systematically described the feasibility of chiral thioene compounds based on sulfur chirality and sulfur coordination as a new chiral ligand in asymmetric reactions. Among them, the chiral sulfenamide-ene research report firstly proposed the design concept of chiral thioene ligands in detail. After the article was published, it was reported by Synfacts (2011, 9, 0985) as the highlight of organic synthesis.
Further research has also successfully designed chiral thioene ligands with a simpler linear molecular framework (a simple one-pot synthesis in two steps, Org. Biomol. Chem. 2012, 10, 1764-1768, cover Article), and used for a more challenging catalytic asymmetric 1,2-addition to construct a series of α-hydroxy acids and α-hydroxy ketones containing quaternary carbon chiral centers, the highest ee value of the product can reach 99%. The research results were published in "German Applied Chemistry" (Angew. Chem. Int. Ed., 2012, 51, 780-783). Among them, the research on the asymmetric 1,2-addition of boric acid to α-diketone compounds is the first report in the world, and the establishment of this method is also the chiral 1,3- The dihydroisobenzofuran skeleton provides a new strategy. This method can be expected to be used for efficient asymmetric synthesis of a series of drug-like molecules.
This series of research work has received strong support from the National Natural Science Foundation of China, the Ministry of Science and Technology and the Chinese Academy of Sciences.
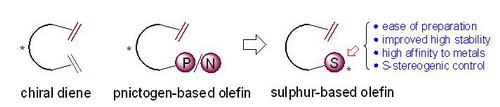
Design of Chiral Thiene Ligand

Org. Biomol. Chem. Magazine cover
Spa Massage Bed,Massage Bed,Wooden Massage Bed,Massage Spa Bed
Kimya Beauty Salon Manufacturer , https://www.jmkimya.com